[] TOCSY
TOCSY (Total Correlation Spectroscopy) creates correlations between all protons within a given spin system, not just between geminal or vicinal protons as in COSY. Correlations are seen between distant protons as long as there are couplings between every intervening proton. This is extremely useful for identifying protons on sugar rings or amino acids: All protons on a given sugar ring will have a correlation with all other protons on the same ring but not with protons on different rings.
Magnetization is transferred successively over up to 5 or 6 bonds as long as successive protons are coupled. Transfer is interrupted by small or zero proton-proton couplings. The presence of hetero-atoms, such as oxygen, usually disrupts TOCSY transfer. The number of transfer steps can be adjusted by changing the spin-lock time. A short time such as 20ms will give only one-step transfers and its TOCSY spectrum will be very similar to its COSY spectrum. More usefully, a long spin-lock time such as 80ms or 120ms will give up to 5 or 6-step transfers. The number of transfers depends on exact coupling details. A useful paper detailing TOCSY transfer in various sugars is Gheysen, K. et. al., Chem. Eur. J. 2008, 14, 8869-8878.
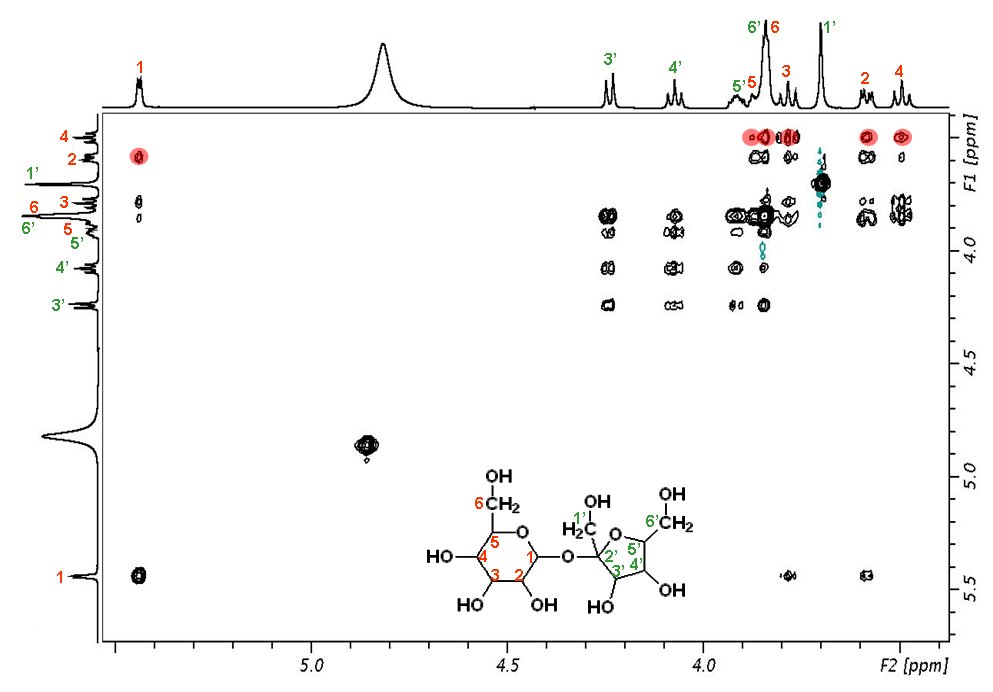
Shown below is a 400 MHz spectrum of sucrose. The red circles show the connections between proton 4 and all the other protons of the glucose ring.
TOCSY (TOtal Correlation SpectroscopY) is a method for correlating many (in theory, all) of this spins in a set of mutually coupled spins. For example, in a polypeptide, TOCSY could identify the set of resonances for each individual amino acid. It is not necessary that all the correlated spins be (directly) coupled to one another, but only that they have common coupling partners. Thus TOCSY can be thought of as a sort of generalized COSY (although the mechanisms are fundamentally different), providing information that complements COSY data.
The key to producing a TOCSY spectrum is to have a period during the pulse sequence (?mixing period?) during which only scalar coupling is acting, while chemical shift is suppressed (not merely refocused). During the mixing period, magnetization is exchanged among coupled spins. The basic process is transfer of magnetization between pairs of coupled spins. For example, in a 3 spin system (AMX) magnetization is exchanged between M and each of its coupling patterns, A and X. However, since this is a continuous process (unlike COSY), once some magnetization has benn passed from A to M, it can be passed further, from M to X; thus, magnetization is passed from A to X, via M. At the same time, magnetization is also passed from X to A, again via M. The amount of magnetization transferred depends on the duration of the mixing period, in a somewhat complicated way in the case of larger numbers of mutually coupled spins.
A variety of schemes can be used to achieve mixing, but the most effective- and therefore now universally used methods involve composite pulse spin locking. The first such method was developed by Bax (who called the experiment HOHAHA), and his method is still entirely adequate in most cases. This method uses the MLEV composite pulse cycle. Newer methods involve different composite pulse spin locking schemes, such as DPSI (developed by Shaka).
The duration of the mixing period depends on two factors, the size of the coupling constants among the various spins in the system, and the number of transfer steps required to see the desired correlations. The basic process of transferring magnetization between to coupled spins requires a mixing time of 1/2J to be complete, although partial transfer (and thus appearance of cross peaks in the 2D spectrum) is achieved in considerably less than this. The relay of magnetization to more remote spins takes longer, though not in an easily predictable way.
Another strategy for making more effective use of TOCSY is to take a series of spectra with different mixing times. Using a mixing time of only 20 msec or so gives a spectrum showing essentially the same correlations as does a COSY spectrum. Taking further spectra with increasing mixing times will show new cross peaks, and different intensities for cross peaks seen in previous spectra. Analysis of the entire series can give a reasonable clear picture of the overall spin system, again circumventing the problems of overlap that may hinder interpretation of the COSY spectrum.
TOCSY
1. Correlations among individual spins in groups of mutually coupled spins
2. Correlations both for pairs of spins actually coupled to one another and for spins within the same group but not directly coupled to each other.
3. Information is complementary to information from COSY
4. All modern TOCSY sequences use a composite pulse spin lock, such as MLEV or DPISI
5. RF field strength during spin lock should be about equal to the spectral width
6. Required power level, and 90o degree pulse width at this level, can be calculated from observe pw90 and tpwr
7. Duration of the spin lock depends both on coupling constants and the number of bonds separating the spins to be correlated.
8. TOCSY is a sensitive experiment (high S/N):
9. Phase sensitive, so can use sensitivity enhancement window functions
10. Cross peaks are in phase (not antiphase), so no cancellation of antiphase multiplet components
11. Cross peak intensity develops during and depends only on the spin lock, not the number of increments or a dephasing delay (as in COSY).
12. Typical mixing times range from 20 to 100 msec
13. Short mix times give COSY ?like spectra: cross peaks primarily for coupled spins
14. Longer mix times show cross peaks for relayed correlations, involving transfer of magnetization between spins not actually coupled each other
15. Longer mix times also allow time for transfer via small coupling constants
A series of spectra with mixing time going from short to long can ?map out? a spin system, and may allow interpretation when the COSY spectrum cannot be fully interpreted.
Finally, TOCSY can also be performed as a 1D experiment, by selective excitation of just one or a few resonances in the spectrum, followed by TOCSY transfer. The resulting (1D) spectra are actually subspectra of the full spectrum, showing just those resonances correlated with the selectively excited peak(s). A series of such 1D experiment can be done in a matter of minutes.
[] NOESY
NOESY experiments work well for molecules of very low and very high molecular weight. They do not work well for molecules with molecular weights of approximately 1000 - 2000 g/mol at typical field strengths, where the NOE's are very close to zero. A ROESY experiment can be used to get NOE information for molecule in this intermediate molecular mass regime. For high molecular weight molecules a NOESY and a ROESY experiment will give very similar results with the exception that the cross peaks will be in phase with respect to the diagonals for a NOESY and 180 degrees out of phase with the diagonals in the case of a ROESY. The figure below shows both a NOESY and a ROESY for gramacidin at 300 MHz The red contours are negative and the black contours are positive.

ref.
http://www.columbia.edu/cu/chemistry/groups/nmr/tocsy.html
http://u-of-o-nmr-facility.blogspot.com/2008/02/noesy-vs-roesy-for-large-molecules.html
'Stage 2 > Structure Idenification (NMR , LCMS)' 카테고리의 다른 글
| Carbonyl (0) | 2010.08.15 |
|---|---|
| HMBC ( Heteronuclear Multiple Bond Correlation ) (0) | 2010.08.15 |
| DEPT NMR (0) | 2010.07.23 |
| NMR operation (0) | 2010.05.26 |
| NMR solvent " chemical shift " (0) | 2010.03.23 |